Basic Tests for the two genes most commonly associated with Hereditary Breast & Ovarian Cancer (BRCA1, BRCA2).

Standard Test covers the 4 genes that account for ~95% of all Hereditary Breast & Ovarian Cancer cases in the Indian population (BRCA1, BRCA2, PALB2, TP53).

Comprehensive Test covers 19 genes (ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53).

Every four minutes, a woman in India is diagnosed with breast cancer. Every eight minutes, a woman loses her life to it. Breast cancer is the most common kind of cancer among women in India.
Ovarian cancer, too, is estimated to be the third most common gynecological cancer in India, one with a very high case to fatality ratio. And experts believe that the rates are only ever set to increase.
Post cancer survival is almost 80% in the US, it is only 60% in India.
One big reason for this is late detection – the survival rate when breast cancer is detected at an advanced stage is nearly three times lower than when it’s caught by Stage I. It’s becoming clear as we learn more about what causes these cancers that family history, indicating inherited gene mutations, is one of the most important risk factors for developing breast and ovarian cancer.
Understanding Hereditary Breast and Ovarian Cancer (HBOC)
Insights from a Senior Genetic Counselor on Life-Saving Tests.
Book a Test
I hereby authorize Strand Life Sciences Pvt Ltd and their certified partners to contact me through email, phone or any other modes of communication. It will override my registry on NCPR.

Strand’s BreastAssure tests use next generation sequencing to reconstruct a person’s genome sequence, and pinpoint any potentially harmful mutations in Hereditary Breast and Ovarian Cancer associated genes. The benefits of next generation sequencing are many and include comprehensive screening, low number of false positives, cost effectiveness and a quick turnover time of a few days.
Frequently Asked Questions
View All
Genetic testing for genes associated with (genes causing)Hereditary Breast and Ovarian Cancer (HBOC) allows screening of those individuals who are at an increased risk of inherited breast cancer and ovarian cancer. For these individuals, counseling, regular monitoring and other cancer prevention strategies can be instituted under the guidance of the Family Physician or Oncologist.
The primary aim of HBOC testing is assess your chances of developing cancer by detecting potentially harmful genetic changes (called bad mutations) in your genes (or DNA). Presence of these mutations does not mean that you will definitely develop cancer. A portion of individuals with the HBOC causing bad mutations develop cancer over their lifetime. Therefore, it is very beneficial to be aware of the presence or absence of HBOC causing bad mutations in your body. Your Family Physician or Oncologist will recommend further regular health checkups.
These tests provide the following benefits:
- Helps in assessment of hereditary cancer risk
- Informs Family Physician or Oncologist in counseling and treatment decisions
- Helps cancer affected individuals and hereditary cancer carriers of harmful mutations in BRCA genes and additional genes in decisions of targeted therapy, such as PARP inhibitors based drugs
- Frequent checks post presence of harmful mutations can help with early detection of cancer can thus help people live longer
By 2025, over 2,00,000 cases of breast cancer and nearly 50,000 cases of ovarian cancer are estimated to be reported annually in India. For women in India there is a 8-12% risk of developing breast cancer and 1.2% risk of developing Ovarian cancer (inclusive of hereditary mutations and non hereditary causes)
6% of breast cancers and 10-20% of ovarian cancers are caused by inherited mutations in the BRCA1 & BRCA2 genes.
As per NCCN/GeneReviews, for BRCA1 mutation carriers, the risk for breast cancer is upto 72% and that for ovarian cancer is up to 58%. For BRCA2 mutation carriers, the risk for breast cancer is upto 69% and that for ovarian cancer, it is upto 29%. The chances of developing breast cancer increase significantly after the age of 35-40 years.
Ideally, as a precaution, every woman over 30 years should be tested for Hereditary Breast & Ovarian Cancer related genetic mutations. However, individuals with a personal or family history of breast or ovarian cancer are predisposed towards HBOC and surely get tested.
Individuals with the following situations should consider this test:
- A personal history of breast or ovarian cancer diagnosed at a young age (premenopausal), bilateral breast cancer (affecting both breasts), or the presence of both ovarian and breast cancer
- A personal history of triple-negative breast cancer below the age of 60 years with or without a family history
- A family history of any of the following cancers at age below 50 years: breast, ovarian, fallopian tube, peritoneal, prostate, or pancreas
- A relative with a known harmful mutation in BRCA1 or BRCA2 genes
- A family history with two or more close relatives with breast or ovarian cancer at any age
- A family history of breast and ovarian cancers in either the same woman or the same immediate family
A harmful genetic variant can be passed onto the next generation from either the maternal or paternal side of the family.
The main difference between the HBOC Basic Test and HBOC Comprehensive Test is the number of genes that are being tested. The HBOC Basic Test covers 4 Genes, while the HBOC Comprehensive Test covers 19 genes. The 4 genes in HBOC Basic Test account for ~95% of all Hereditary Breast & Ovarian Cancer cases in the Indian population. The HBOC Comprehensive test increases the coverage further.
Following are the genes covered by each of these tests:
- HBOC Basic Test: BRCA1, BRCA2, PALB2, TP53
- HBOC Comprehensive Test: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53
BRCA1 (BReast CAncer gene 1) and BRCA2 (BReast CAncer gene 2) are genes that produce proteins that help repair damaged DNA. Everyone has two copies of each of these genes—one copy inherited from each parent. BRCA1 and BRCA2 are sometimes called tumor suppressor genes because when they have certain changes, called harmful (or pathogenic) variants (or mutations), cancer can develop.
People who inherit harmful variants in one of these genes have increased risks of several cancers—most notably breast and ovarian cancer, but also several additional types of cancer. People who have inherited a harmful variant in BRCA1 and BRCA2 also tend to develop cancer at younger ages than people who do not have such a variant.
A harmful variant in BRCA1 or BRCA2 can be inherited from either parent. Each child of a parent who carries any mutation in one of these genes has a 50% chance (or 1 in 2 chance) of inheriting the mutation. Inherited mutations—also called germline mutations or variants—are present from birth in all cells in the body.
Even if someone has inherited a harmful variant in BRCA1 or BRCA2 from one parent, they would have inherited a normal copy of that gene from the other parent (that’s because in most cases, embryos with a harmful variant from each parent cannot develop). But the normal copy can be lost or changed in some cells in the body during that person’s lifetime. Such a change is called a somatic alteration. Cells that don’t have any functioning BRCA1 or BRCA2 proteins can grow out of control and become cancerous.
Positive result: This means that a person has a potentially harmful mutation in one of the breast cancer associated genes. This means that they have a higher risk of getting breast or ovarian cancer than anyone in the general population.. However, a positive test result doesn’t mean that someone will surely develop cancer.
Negative result: This means that no wrong mutation was found. The absence of a specific disease-causing variant can be most reassuring. A negative result does not mean that there is no cancer risk, but rather that the risk is probably the same as the cancer risk in the general population. Even if the report is negative, some individuals may still benefit from increased surveillance depending on the person’s family history and any other risk factors they may have.
Ambiguous result: The results of a genetic test can be classified as ‘Ambiguous’ when a mutation or change is noticed but the significance is unknown based on the scientific data available at the time of reporting. This kind of test result can be called “unclear,” or it can be called a “genetic variant of uncertain significance.” A person with such test results may request for the data to be re-analysed on a periodic basis. Each time the data can be reassessed and re-classified for the presence of any variants that may be newly linked to established genes associated with hereditary cancer or to newly identified disorders since the date of this report.
No, a positive test result does not necessarily mean a person will be affected with cancer. It only increases a person’s risk of developing cancer in a lifetime, when compared to the general population.
No, a positive genetic test result does not, by itself, mean a person has cancer. It means that the person has a genetic variation which makes him/her more susceptible to develop cancer when compared to the general population
No, a positive test result does not necessarily mean a person will be affected with cancer. It only increases a person’s risk of developing cancer in a lifetime, when compared to the general population.
A positive test result may also have important implications for family members, including future generations. It is recommended that they too get tested for these variations.
- Both men and women who inherit a harmful BRCA1 or BRCA2 variant, whether or not they develop cancer themselves, may pass the variant to their children. Each child has a 50% chance of inheriting a parent’s variant.
- All blood relatives of a person who has inherited a harmful BRCA1 or BRCA2 variant are at some increased risk of having the variant themselves. For example, each of that person’s full siblings has a 50% chance of having inherited the variant as well.
- Very rarely, an individual may test positive for a harmful variant not inherited from either parent. Such a variant is one that arose in a germ cell (sperm or egg) of one of the parents and is present in all the cells of the person who grew from that cell. The children of someone with a de novo variant (but not his or her siblings) are at risk of inheriting the variant.
Anyone with a ‘Positive’ result in a Hereditary Breast & Ovarian Cancer (HBOC) genetic test should consult with a Medical Practitioner (Gynecologist, Medical Oncologist etc) to determine subsequent increased surveillance (frequent checks). They may suggest increased surveillance through breast MRI, annual mammography, and semi-annual clinical breast exam and other precautionary interventions.
The direct medical harms of genetic testing are minimal, but knowledge of test results, whether positive or negative, may have harmful effects on a person’s emotions, social relationships, finances, and medical choices.
Dealing with uncertainty of an uninformative negative or a VUS test result is another potential harm. For this reason, it is important to have genetic counseling before undergoing genetic testing.
Results of genetic tests are normally included in a person’s medical records, particularly if a doctor or other health care provider has ordered the test or has been consulted about the test results. Therefore, people considering genetic testing must understand that their results may become known to other people or organizations that have legitimate, legal access to their medical records, such as their insurance company or employer, if their employer provides the patient’s health insurance as a benefit.
Strand Life Sciences is a global genomic profiling company and leader in precision medicine diagnostics, aimed at empowering genetic testing for inherited diseases. For 15 years, our genomics products and solutions have facilitated the work of leading researchers and medical geneticists in over 2,000 laboratories and 100 hospitals around the world.
Strand uses Next Generation Sequencing (NGS) based genomics and DNA analysis to identify multiple harmful mutations. Strand sequencing covers a wide range of mutation types and has high accuracy and sensitivity.
All payments are to be strictly done in electronic format using the payment gateway on our site (BreastAssure Test)
Event
View All

25 October, 2024
Integrating Genomic Testing for Cancer in Gyn...
Join us for an insightful webinar on “Integrating Genomic Testing in Gynecol...
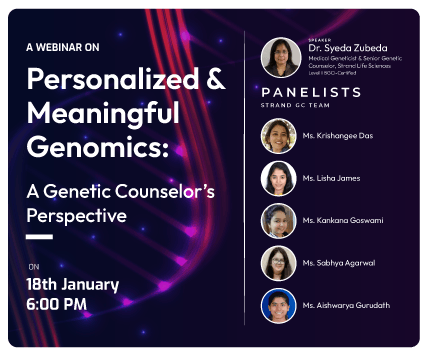
18 January, 2024
Personalized & Meaningful Genomics...
Join our webinar – ‘Personalized & Meaningful Genomics: A Genetic ...
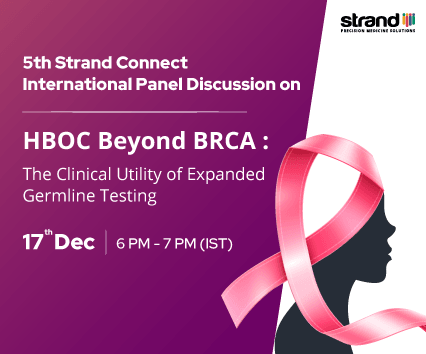
17 December, 2023
HBOC Beyond BRCA...
Join us for an insightful discussion on “HBOC Beyond BRCA: The Clinical Util...
25 November, 2023
Role of Liquid Biopsy in NSCLC...
4th Strand Connect International Panel Discussion on Role of Liquid Biopsy in NSCL...
View All