Introduction
Ovarian cancer remains one of the most challenging cancers to treat, primarily due to its late diagnosis and high recurrence rate. In 2020, global statistics indicated that approximately 310,000 women were diagnosed with ovarian cancer, a disease that resulted in the loss of 200,000 lives and left over 750,000 women living within five years of their diagnosis. Despite these daunting figures, recent advancements in understanding the molecular mechanisms of ovarian cancer have discovered targetable therapeutic pathways.
Homologous Recombination Deficiency
Homologous Recombination Deficiency (HRD) is characterized by the cells’ inability to repair DNA double-strand breaks through the homologous recombination (HR) pathway, a process essential for maintaining genomic stability. Consequently, cancer cells must resort to error-prone repair mechanisms, leading to genomic instability and heightened sensitivity to certain therapies such as PARP inhibitors.
Diagnostic Approaches
The assessment of HR Deficiency is pivotal in identifying optimal treatment strategies, particularly the use of poly-ADP ribose polymerase inhibitors (PARPi), which specifically target HR-deficient tumors. HRD is measured by analyzing mutations in BRCA1 & BRCA2 genes, along with Genomic Scar Score, which is a function of Loss of Heterozygosity (LOH), Telomeric Allelic Imbalance (TAI) and Large Scale State Transitions (LST), which are genomic events that contribute to genomic instability.
Genomic Scar Score (GSS Score)
The GSS Score consolidates genomic instability metrics into a single measure, facilitating the identification of candidates for PARPi therapies. A GSS Score of 50 and greater is termed as “HRD positive”, and indicates benefit from PARPi treatment.
Insights from 900+ HRD Tests
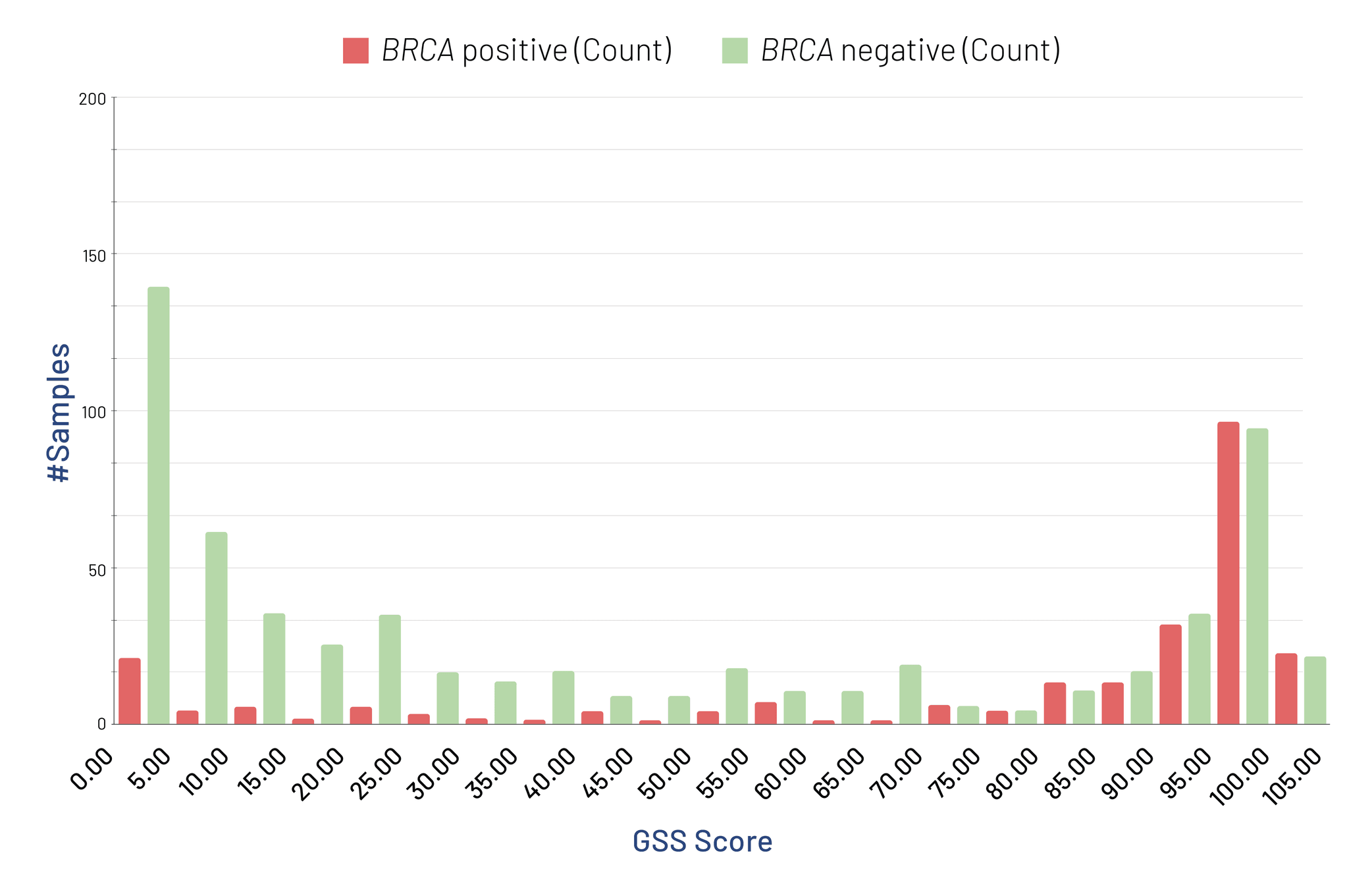
Our analyses of over 970 samples have revealed that more than 52% exhibit high genomic instability, with most BRCA-positive samples also presenting with a high GSS Score. Approximately 57% of these samples are HRD Positive, with 29% qualifying based solely on a high GSS Score, in the absence of a BRCA mutation.
Current Treatment Approaches
Treatment approaches for advanced ovarian cancer encompass a range of surgical and pharmacological interventions.
First-Line Treatment
- Primary Debulking Surgery (PDS) followed by Platinum-Paclitaxel Chemotherapy: This approach involves surgery to remove as much of the tumor as possible. Following the surgery, patients receive chemotherapy with drugs like platinum and paclitaxel, which is considered the standard of care.
- Neoadjuvant Chemotherapy (NACT) followed by Interval Debulking Surgery (IDS): For cases with extensive tumor spread, chemotherapy is administered first to shrink the tumor. After the tumor has been reduced, interval debulking surgery (IDS) is performed to remove the remaining tumor.
- Addition of Bevacizumab (Bev): Bev, an anti-angiogenic drug, is used to prevent the tumor from forming new blood vessels. Integrating Bev into the standard chemotherapy regimen has been shown to improve outcomes.
- Upfront HRD Testing: Upfront testing for HRD is being increasingly adopted for the management of advanced ovarian cancers. In the PRIMA study, which evaluated the progression-free survival of newly diagnosed advanced ovarian cancer, 59% of the patients who had tumors with homologous-recombination deficiency had no disease progression or decrement in quality of life.
Second-Line Treatment
Despite advancements in first-line treatments, approximately 70% of patients with advanced ovarian cancer will experience relapses. Second-line treatment options include additional chemotherapy, targeted therapies like PARPi, and immunotherapy, aiming to control the disease and enhance quality of life. The use of PARPi like Bev post-chemotherapy necessitates careful management of tolerance issues, often favoring PARPi monotherapy over combination therapy to optimize efficacy and manage side effects.
Future Prospects
Advancements in genomic technologies and bioinformatics are anticipated to enhance HRD diagnostic and prognostic tools. Approvals for HRD testing may also expand beyond ovarian cancer to other malignancies that exhibit similar DNA repair deficiencies, such as breast and prostate cancer. Ongoing research aims to validate the use of HRD testing across a wider range of cancers, potentially paving the way for targeted therapies in these tumors.
References:
- Reid F, Bajwa A, Assistant R. THE WORLD OVARIAN CANCER COALITION ATLAS 2023 GLOBAL TRENDS IN INCIDENCE, MORTALITY, AND SURVIVAL. 2023.
- Heitz F, Ataseven B, Staniczok C, Denkert C, Rhiem K, Hahnen E, et al. Implementing HRD testing in routine clinical practice on patients with primary high-grade advanced ovarian cancer. Cancers (Basel). 2023;15(3):818.
- Kekeeva T, Andreeva Y, Tanas A, Kalinkin A, Khokhlova S, Tikhomirova T, et al. HRD testing of ovarian cancer in routine practice: what are we dealing with? Int J Mol Sci. 2023;24(13):10497.
- González-Martín, A., Pothuri, B., Vergote, I., DePont Christensen, R., Graybill, W., Mirza, M. R., … & Monk, B. J. (2019). Niraparib in patients with newly diagnosed advanced ovarian cancer. New England Journal of Medicine, 381(25), 2391-2402.
